Navigating the regulatory landscape for using Altosid® IGR (S)-Methoprene in cattle feed can be challenging. Understanding the differences in regulatory status between the US and Canada and ensuring compliance with guidelines from the EPA and other regulatory agencies are crucial steps for the successful use of this feed additive. This blog aims to provide clarity on Altosid® IGR regulations.
(S)-Methoprene Regulatory Status
(S)-Methoprene, the active ingredient in Altosid® IGR, is an insect growth regulator (IGR) effective against various pests such as fleas, mosquitoes, horn flies, ants, sciarid flies, and stored product pests. The EPA first registered (S)-Methoprene in 1975, classifying it as the first biochemical pesticide. This classification places it under the regulation of the Biopesticide Division of the EPA.
In 1991, (S)-Methoprene underwent an extensive reregistration process, culminating in the publication of the Reregistration Eligibility Document (RED). In June 2003, the EPA announced an exemption for (S)-Methoprene from the requirement of a tolerance in or on all food commodities when used to control insect larvae. This exemption was granted after a thorough risk analysis determined that residue tolerances were unnecessary to protect human health or the environment.
Discussions between the EPA and FDA resulted in the decision that the feed-through uses of (S)-Methoprene in cattle feed to control horn flies should be regulated by the EPA. Consequently, Altosid® IGR Feed-Thru became the first (S)-Methoprene cattle product registered by the EPA in 1975.
Regulatory Status of Altosid® IGR Feed-Thru
The 2015 Feed Additive Compendium provides a detailed summary of the regulatory status for the use of (S)-Methoprene in cattle feed. Key points include:
- FDA Status: No feed mill license is required for (S)-Methoprene, and it is classified as a food additive under 40 CFR 180.1033. (S)-Methoprene is exempt from tolerance requirements when used to control insect larvae. When included in medicated feeds, the application requirement is determined by the regulatory status of the drug.
- EPA Status: (S)-Methoprene is considered a pesticide when used in non-medicated feeds, requiring EPA registration for feeds offered for sale unless custom-blended per the provisions of 40 CFR 167.3. In medicated feeds, (S)-Methoprene is treated as a feed additive, and no EPA registration is needed if the source product is EPA-registered.
Understanding the Differences in Regulatory Status Between the US and Canada
In Canada, the Pest Management Regulatory Agency (PMRA) oversees the regulation of pesticides similar to the EPA in the US. On January 4, 2016, the PMRA approved the Altosid® 2% MUP product. The Canadian Feed Ingredient Association (CFIA) is responsible for regulating end-use feeds containing Altosid® IGR. These Altosid® IGR Canada regulations are crucial for compliance in the Canadian market.
While both the US and Canada have similar regulatory frameworks, it is essential to note the specific requirements and processes in each country. For instance, while the EPA handles the registration and exemption processes in the US, the PMRA and CFIA are the corresponding agencies in Canada. Understanding these differences ensures compliance and smooth navigation through the regulatory requirements in both countries.
Ensuring Compliance with EPA and Other Regulatory Agencies' Guidelines
To ensure compliance with EPA and other regulatory agencies' guidelines for feed additives like Altosid® IGR, follow these steps:
- Stay Informed: Regularly check updates from the EPA, FDA, PMRA, and CFIA regarding changes and guidelines for (S)-Methoprene and other cattle feed additive regulations.
- Documentation: Maintain thorough documentation of all regulatory submissions, approvals, and correspondences with regulatory agencies.
- Custom-Blending: If custom-blending feed products, ensure compliance with provisions such as 40 CFR 167.3 to avoid unnecessary registrations.
- Labeling and Usage: Adhere to the labeling requirements and usage instructions provided by the EPA and other regulatory bodies to avoid violations.
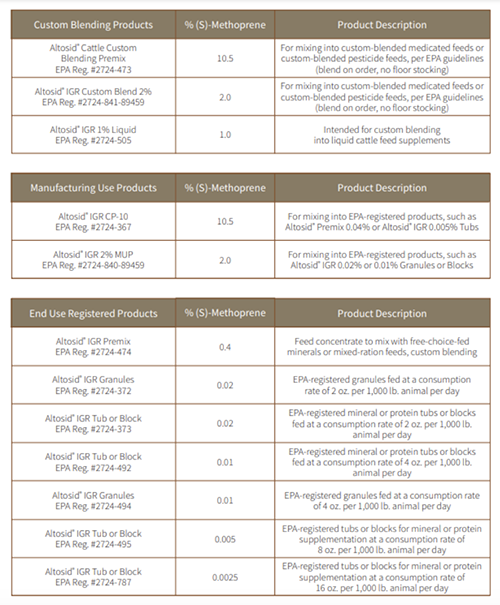
Altosid® IGR Products for Horn Fly Control
Altosid® products are registered for cattle (beef and dairy) for continuous feeding during the fly season to prevent the breeding of horn flies in the manure of treated cattle at the level 1.13 mg/cwt/day.